Thousands of men in England with advanced prostate cancer are set to gain access to a new life extending treatment following a decision by the National Institute for Health and Care Excellence. The move marks a significant development in the management of one of the most common cancers affecting men and reflects growing progress in precision oncology.
A spokesperson from Prostate Cancer UK said:
“For many men with advanced prostate cancer, treatment options can feel limited. Expanding access to innovative therapies like this is a vital step in improving both survival and quality of life.”
Dr Samantha Roberts, Chief Executive of NICE, said:
“Our recommendation means thousands of men across England will be able to benefit from a clinically effective treatment that represents good value for the NHS.”
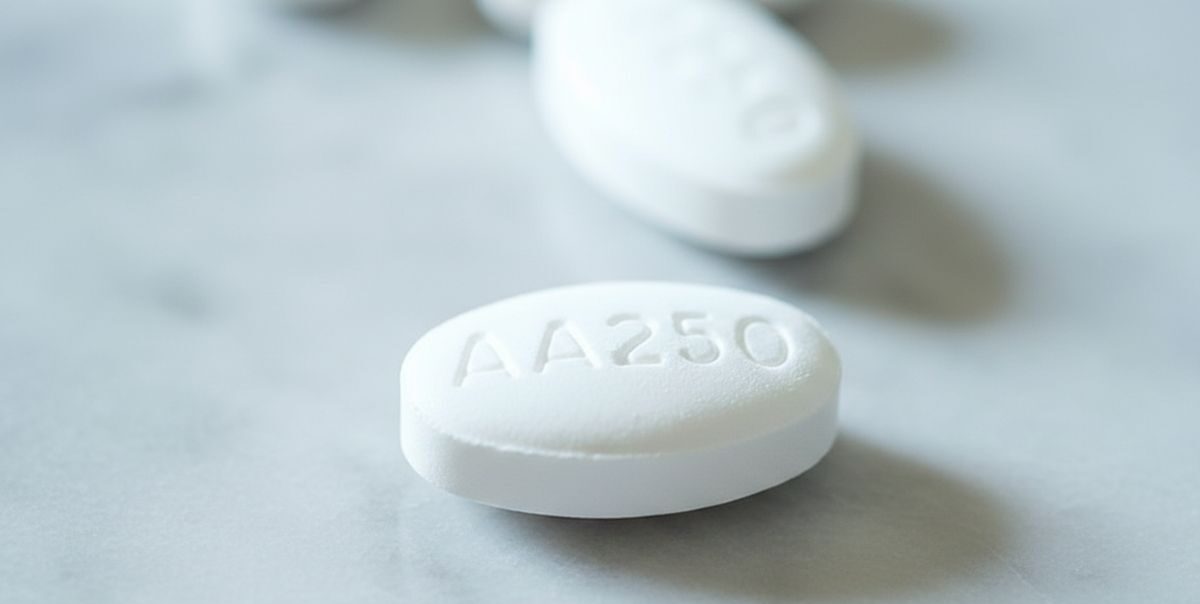
The treatment involves the targeted cancer drug olaparib used in combination with the hormone therapy abiraterone for men with advanced prostate cancer that has spread beyond the prostate and no longer responds to standard hormone treatments. Clinical evidence shows the combination can significantly delay disease progression and extend survival for eligible patients.
A shift in treatment options
Prostate cancer is the most common cancer in men in England, with advanced stages often proving difficult to treat once the disease becomes resistant to hormone therapy. Until recently, treatment options for men with metastatic castration resistant prostate cancer were limited, and outcomes were often poor.
The newly approved combination therapy works by exploiting weaknesses in cancer cells’ ability to repair damaged DNA. Olaparib belongs to a class of medicines known as PARP inhibitors, which block DNA repair pathways and cause cancer cells to die. When combined with abiraterone, which suppresses androgen production, the therapy has been shown to slow tumour growth more effectively than hormone treatment alone.
Clinical evidence and survival benefit
Clinical trial data reviewed by NICE demonstrated that men receiving the combination therapy experienced a substantial delay in cancer progression compared with those receiving standard treatment. Importantly, the benefit was observed across a broad group of patients, not only those with inherited genetic mutations previously associated with PARP inhibitor response.
Oncologists say the approval represents a meaningful advance because it offers patients additional time with controlled disease, potentially improved quality of life and fewer complications associated with late stage progression.
Impact on patients and the NHS
NICE estimates that thousands of men in England could be eligible for the treatment each year. The decision follows confidential commercial negotiations that made the drug combination cost effective for use within the NHS.
Cancer specialists have welcomed the move, noting that it aligns England with other countries where the therapy is already available. Patient advocacy groups have also highlighted the importance of timely access to innovative treatments, particularly for conditions where therapeutic options have historically been limited.
Professor Johann de Bono, Consultant Medical Oncologist at The Royal Marsden, said:
“This decision gives men with advanced prostate cancer earlier access to a targeted treatment that can meaningfully delay progression of the disease and extend survival.”
Precision medicine in prostate cancer
The approval reflects a broader shift toward precision medicine in prostate cancer care. Rather than relying solely on traditional chemotherapy or hormone therapy, clinicians are increasingly using targeted drugs based on tumour biology and molecular vulnerabilities.
Research continues into how best to sequence treatments and identify which patients are most likely to benefit. Ongoing studies are also exploring whether similar drug combinations could be used earlier in the disease course or alongside emerging therapies.
Looking ahead
While the new treatment is not a cure, experts say it represents an important step forward in extending survival and improving outcomes for men with advanced prostate cancer. As more targeted therapies enter clinical practice, the outlook for patients is gradually improving.
The decision also underscores the role of NICE in balancing innovation with affordability, ensuring that life extending medicines can reach patients while remaining sustainable for the health system.