Today, oncology drug development continues to experience the highest attrition rates across therapeutic areas[1]. Despite major advances in molecular profiling and biomarker-driven strategies, a significant proportion of oncology clinical trials still fail, often due to challenges in patient selection.
Many trials rely on tissue biomarkers that are weak, noisy, or insufficiently predictive of treatment response. In routine practice, these biomarkers are frequently assessed using manual or semi-quantitative scoring methods that are inherently subjective, difficult to reproduce and prone to inter- and intra-observer variability. As a result, patient identification can be inconsistent across sites, compromising trial integrity and outcomes.
Significant variability persists in pathology analysis, even with established international guidelines, standardized protocols, and external quality control. A well-documented example is the Ki-67 proliferation index. Research from the Karolinska Institutet[2] in Sweden demonstrated that while the median Ki-67 value across laboratories was 22%, reported values ranged from 15% to 30%. Moreover, the majority of laboratories showed substantial year-to-year variation. Such discrepancies can directly influence patient eligibility and stratification in clinical trials.
These challenges are further exacerbated by a global shortage of pathologists and a rising incidence of cancer, increasing diagnostic workloads and placing additional pressure on pathology services[3]. Together, these factors contribute to delays, variability and uncertainty in biomarker-driven decision-making.
Beyond variability, there is a second, deeper limitation. Many patients differ as responders or non-responders based on subtle morphological patterns that are difficult or impossible to discern with the human eye alone. Traditional approaches often reduce complex tissue biology to a single score, potentially losing critical contextual information.
When biomarker quantification is subjective, inconsistent and unable to capture biological complexity, clinical decision-making may be compromised, increasing the risk of late-stage trial failure. This raises a central question for clinical research and drug development: how can complex biomarker decisions be made feasible, consistent, scalable and trustworthy?
The Role of AI-Enabled Pathology in Clinical Research
AI-enabled pathology offers a compelling answer. Rather than replacing expert judgment, AI augments pathologists with objective, quantitative and reproducible biomarker assessments at scale. By analysing whole-slide images consistently, AI reduces inter- and intra-observer variability and captures tumour heterogeneity more comprehensively than manual evaluation alone. This enables higher efficiency, improved standardisation, and more reliable patient selection in clinical trials[4].
Evidence supporting this approach is rapidly accumulating. At ASCO last year, Mindpeak presented results from an international study conducted in collaboration with AstraZeneca focusing on HER2-ultralow breast cancer[5]. The study included 1,940 HER2 IHC cases reviewed by 105 expert pathologists across ten countries, reflecting real-world trial conditions. AI support acted as a safety net for biomarker assessment, reducing misclassification of HER2-ultralow samples incorrectly scored as null from 30.5% to 4.5%.
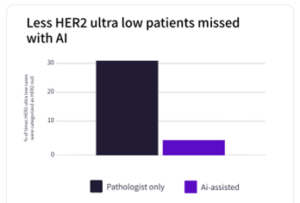
For clinical research and drug development teams, the implications are tangible: fewer misclassified patients, improved reproducibility across sites and reduced risk of trial failure. AI-enabled pathology allows teams to pressure-test complex biomarker strategies earlier in development, mitigating late-stage risk and supporting more informed decision-making.
From Simplicity to Biological Complexity
Crucially, AI is also changing how complexity is handled in pathology. Complexity is no longer the output; it is the input. AI-based tissue analysis is no longer experimental; it is increasingly clinically validated.
Mindpeak IMS
A prominent example is the Phase III TROPION-Lung01 trial.[6] Traditional biomarker methods failed to accurately predict patient response to anti-TROP2 ADCs. To address this limitation, researchers developed a novel, AI-driven Quantitative Spatial Computational pathology score called QCS from whole-slide images, which significantly advanced conventional IHC scoring. This approach went beyond conventional IHC scoring and proved predictive of clinical outcomes, contributing to the success of this late-stage trial.
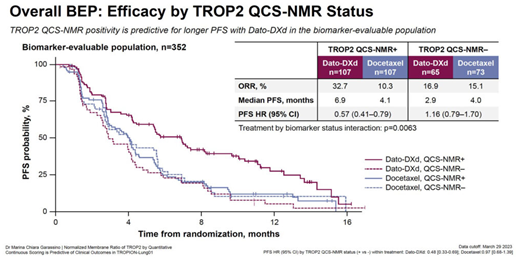
Such examples illustrate that the integration of AI-augmented scoring with existing IHC assays is already occurring in practice. These approaches enable more precise patient–drug matching by identifying responders that might otherwise be missed.
From Innovation to Implementation
The strategic question is no longer whether AI should be used in clinical trials, but how to implement it effectively. Translating promising AI-derived biomarkers into clinical and commercial success requires more than algorithmic performance.
In the context of deploying AI within clinical trials, and ultimately in routine clinical practice, performance alone is not sufficient. What determines whether an AI solution can be adopted, trusted, and scaled is regulatory readiness. AI-based tools must successfully undergo rigorous clinical, technical and regulatory scrutiny in real-world settings to demonstrate safety, reliability, and clinical utility. Regulatory approval expertise, therefore, should therefore not be viewed as an afterthought, but as a core pillar in the development of AI-enabled pathology solutions.
Beyond technical validation and regulatory approval, system-level readiness is equally critical. Widespread adoption of AI in pathology also depends on the ability of regulatory authorities and reimbursement frameworks to appropriately evaluate, recognise and support AI-driven technologies. Clear regulatory pathways, transparent evaluation criteria and aligned reimbursement policies are essential to enable sustainable integration of AI into clinical workflows and ensure that innovation can translate into real patient and healthcare system benefit.
Finally, AI decision-support tools must also integrate seamlessly into existing digital pathology workflows. Successful deployment requires compatibility with the scanners, viewers and reporting environments pathologists already use. Integration, not disruption, is key. That means validated models, regulatory-ready software, secure deployment and intuitive user interfaces that support, rather than replace, expert judgment. When deployed in this way, AI becomes a shared language between R&D, translational teams and clinical pathology, enabling consistency from early discovery all the way to patient care.
Looking Ahead, From Diagnosis to Decision Intelligence
AI is no longer a mere technology option in drug development; it’s a strategic lever to de-risk late stage-clinical trials. As clinical trials grow more complex and precision medicine demands ever more accurate patient stratification, the limitations of subjective, manual biomarker assessment are becoming increasingly difficult to justify.
Frontier AI models make it possible to unlock the full data potential of pathology slides, identifying spatial patterns and complex biological interactions that are otherwise inaccessible.
AI-enabled pathology represents a shift from static, single-marker readouts to dynamic, data-rich decision support. By integrating spatial context, tissue architecture and complex biological interactions, AI transforms pathology slides into quantitative assets that can inform trial design, patient selection and endpoint assessment with unprecedented consistency and scale.
Crucially, the true impact of AI will not be determined by algorithms alone. Its success depends on thoughtful integration into clinical workflows, robust regulatory validation and healthcare systems that are prepared to evaluate and reimburse AI-driven decision-support tools. When these elements align, AI becomes more than a technological upgrade, it becomes an enabler of trust across research, clinical and regulatory stakeholders.
Looking ahead, pathology is evolving from a retrospective diagnostic discipline into a proactive engine for translational insight. AI has the potential to bridge discovery and clinical development, allowing biomarkers to be explored, validated and operationalised within a single, continuous framework.
In this future, clinical trials are no longer constrained by the limits of human perception or inter-site variability. Instead, they are guided by reproducible, scalable and biologically meaningful insights, turning uncertainty into informed decision-making.
AI-enabled pathology is not replacing expertise; it is amplifying it, and in doing so, helping to redefine how precision medicine is delivered.
Author Profile:
Aurélie is Vice President of Predictive AI Solutions at Mindpeak, where she leads the strategy to shift pathology from descriptive to predictive approaches, supporting therapy response identification, treatment decision-making, and accelerated drug development. She brings over 15 years of experience across AI-driven precision medicine, biomarkers, and commercial strategy, with senior roles at Owkin, Veracyte, and QIAGEN. Her background combines deep expertise in biomarkers, pharma business development, and product strategy with a strong ability to translate complex scientific and AI concepts into clear business and clinical value propositions.