By early Q4 2025, global pharma M&A had already crossed $70B in announced value, driven heavily by a handful of mega-deals, more than 2024, and tracking toward the highest levels since the post-2015 boom. Unlike 2023–24, this wave isn’t driven by small tuck-ins....
Pharma 5.0: The Future of Human-Centric, Intelligent, Sustainable Medicine
As the pharmaceutical industry undergoes rapid transformation, a new paradigm is taking shape. Known as Pharma 5.0, it represents more than an upgrade in technology, it is a fundamental shift toward human-centric innovation, sustainable manufacturing, and intelligent...
Autolus CAR-T Offers Hope to Adults with Aggressive Blood Cancer
The National Institute for Health and Care Excellence (NICE) has published final draft guidance recommending a novel, next-generation chimeric antigen receptor T-cell (CAR-T) therapy for use in the National Health Service (NHS). This landmark decision is set to...
Decision Frameworks in Pharma
Decision Frameworks in Pharma: From RAVE and DICE to Lilly’s Truth-Seeking Machine What Works, What Kills What, and Why It Matters In pharmaceutical R&D, where billions ride on the flip of a biological coin, decision frameworks aren’t just nice-to-haves - they’re...
Follow the Science? It Would Rather Follow a Leader… (2025 Update)
Eight years on from the first version of this article, “follow the science” is still the industry’s favourite comfort blanket. It sounds noble, unassailable, the kind of thing you put on a lab-wall poster next to a photo of Rosalind Franklin. (Following the X...
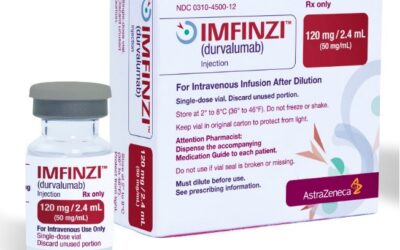
Durvalumab approved in the US
Durvalumab approved in the US as first & only perioperative immunotherapy for patients with early gastric & gastroesophageal cancers! The FDA approved Durvalumab (Imfinzi) in combination with Fluorouracil, Leucovorin, Oxaliplatin, and Docetaxel (FLOT)...
No Results Found
The page you requested could not be found. Try refining your search, or use the navigation above to locate the post.