Latest News
The Convergence Era: How Data and AI are Reshaping Life Sciences
For decades, innovation in life sciences followed a familiar path: lab discovery, clinical testing, regulatory approval, market release. It was linear. Predictable. Slow. What’s happening now feels different. The most significant shift isn’t a single breakthrough...
Latest News

GMCA: Why Unlocking Longevity Research Will Drive Innovation
The science of longevity has never been more active. With advances in preventive medicine and digital health, as well as new insights into lifestyle, environment, drug therapies and genetics, there is more research available than ever on how people age and live well....

Understanding GLP-1 Side Effects Through a Nutritional Lens
GLP-1 medications have changed the landscape of weight management. For many people, they have delivered clinically meaningful weight loss alongside improvements in glycaemic control, and their place in treatment pathways is now well established. What is becoming...

PICTURE: Democratising Clinical Intelligence from the Frontline
A New Era for EHR Data A new study published in the latest issue of the Royal College of Physicians’ Future Healthcare Journal introduces PICTURE, an innovative data platform designed to convert routine electronic health record (EHR) data into actionable clinical...

European Life Sciences: Rethinking the “Organic” US Expansion
Against a backdrop of rising development and launch costs, growing regulatory complexity, geopolitical uncertainty and intensifying competition for specialist talent, many European pharma and biotech companies are reassessing the long-held ambition of “cracking the...
Join The Future Of Life Sciences Media
In a world overflowing with information, LifeScienceDaily.news stands apart as the dedicated space where life sciences professionals share what’s new, what’s next, and why it matters — all in real time.
Don’t wait for the media to pick up your story. Be the media.
Trending
More News
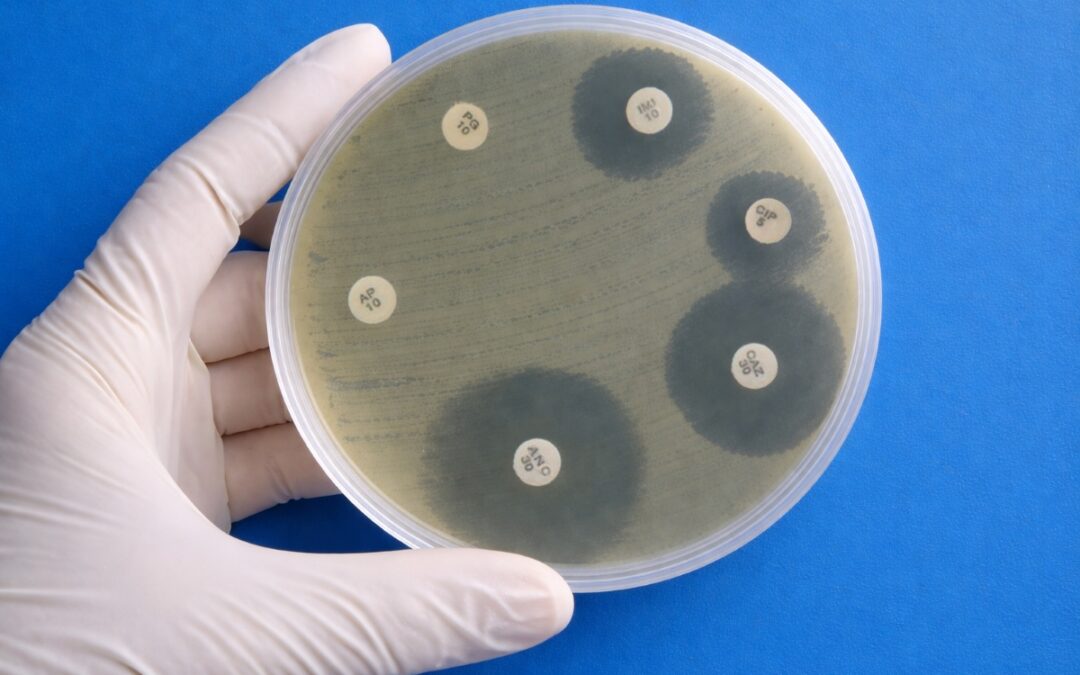
Antibiotic Resistance: The Silent Pandemic Undermining Modern Medicine
Antibiotics are one of the greatest success stories in modern medicine. They transformed once-deadly infections into treatable illnesses, made routine surgery possible, and helped extend human life expectancy worldwide. From treating pneumonia to protecting patients...

Food Allergy Risk is Rising Faster Than Our Systems Can Respond
Why Life Sciences and Health Tech Must Rethink Prevention Food allergies represent a growing and under addressed challenge within public health and preventative medicine. While prevalence and severity continue to rise, the systems designed to protect individuals have...

Raising Expectations for Medical Liaisons
In the last decade, the Medical Science Liaison role has evolved from a scientific messenger to a field based strategist operating in one of the most highly scrutinized environments in healthcare. That evolution is why board certification is moving from optional...

Lilly Finalizes “Lilly Lehigh Valley” with $3.5B Manufacturing Hub
Eli Lilly and Company has officially selected Fogelsville, Pennsylvania, as the site for its newest $3.5 billion injectable medicine and device manufacturing facility. Operating under the name "Lilly Lehigh Valley," the project represents the final piece of a quartet...

Why Collagen Has Become the Cornerstone of Modern Aesthetics
Beauty is no longer driven by illusion. The era of exaggerated results and quick fixes is giving way to a more intelligent, informed approach, one that prioritises biology over bravado. At the heart of this shift sits collagen, a once-overlooked structural protein now...

AstraZeneca Commits $15bn to Expand R&D and Manufacturing in China
AstraZeneca has announced plans to invest up to 15 billion US dollars to expand its research, development, and manufacturing operations in China, reinforcing the country’s role as a central pillar of the company’s global strategy. The long-term investment will fund...

Why Hypoxia Workstations are Becoming Standard Lab Infrastructure
“Hypoxia” used to be nothing but a niche biology topic. Today it is quietly becoming standard infrastructure in labs. Two forces are converging. Regulators and funders are nudging R&D away from default animal studies and toward human-relevant evidence. At the...

Our Reliance on Antidepressants: What the Numbers Are Telling Us
Antidepressant use has become a defining feature of modern mental healthcare. In the UK alone, an estimated 8.7 million people are currently taking antidepressant medications, reflecting both the scale of mental health need and the central role pharmacological...

Phacilitate Unites CGT Community for New Era Defined by Collaboration
Phacilitate announces the return of Advanced Therapies Week (ATW) for its 22nd year, reinforcing its status as one of the most established and influential cell and gene therapy (CGT) conferences in the United States and among the most recognized globally. In...

Natural Product Drug Discovery Returns With New Anti Cancer Insights
Scientists have uncovered how plants produce mitraphylline, a rare natural compound with demonstrated anti cancer properties, shedding new light on the biological machinery behind one of nature’s most complex chemical products. By identifying the key enzymes involved...

Biotech’s Funding Winter is Over, but M&A Will Remain Selective
Biotech is showing early signs of life after a prolonged funding winter, but for many early-stage companies, conditions remain unforgiving. Years of low valuations, high borrowing costs and regulatory uncertainty have reshaped investor behaviour, with capital scarce...

Thousands Benefit From New Treatment for Advanced Prostate Cancer
NICE recommends life-extending daily pill which offers hope for people who cannot take standard treatment. Thousands of people living with advanced prostate cancer will have access to a life-extending new treatment that can be taken at home from today (Friday, 23...

Antibiotic Resistance: The Silent Pandemic Undermining Modern Medicine
Antibiotics are one of the greatest success stories in modern medicine. They transformed once-deadly infections into treatable illnesses, made routine surgery possible, and helped extend human life expectancy worldwide. From treating pneumonia to protecting patients...

Food Allergy Risk is Rising Faster Than Our Systems Can Respond
Why Life Sciences and Health Tech Must Rethink Prevention Food allergies represent a growing and under addressed challenge within public health and preventative medicine. While prevalence and severity continue to rise, the systems designed to protect individuals have...

Raising Expectations for Medical Liaisons
In the last decade, the Medical Science Liaison role has evolved from a scientific messenger to a field based strategist operating in one of the most highly scrutinized environments in healthcare. That evolution is why board certification is moving from optional...

Lilly Finalizes “Lilly Lehigh Valley” with $3.5B Manufacturing Hub
Eli Lilly and Company has officially selected Fogelsville, Pennsylvania, as the site for its newest $3.5 billion injectable medicine and device manufacturing facility. Operating under the name "Lilly Lehigh Valley," the project represents the final piece of a quartet...

Why Collagen Has Become the Cornerstone of Modern Aesthetics
Beauty is no longer driven by illusion. The era of exaggerated results and quick fixes is giving way to a more intelligent, informed approach, one that prioritises biology over bravado. At the heart of this shift sits collagen, a once-overlooked structural protein now...

AstraZeneca Commits $15bn to Expand R&D and Manufacturing in China
AstraZeneca has announced plans to invest up to 15 billion US dollars to expand its research, development, and manufacturing operations in China, reinforcing the country’s role as a central pillar of the company’s global strategy. The long-term investment will fund...

Why Hypoxia Workstations are Becoming Standard Lab Infrastructure
“Hypoxia” used to be nothing but a niche biology topic. Today it is quietly becoming standard infrastructure in labs. Two forces are converging. Regulators and funders are nudging R&D away from default animal studies and toward human-relevant evidence. At the...

Our Reliance on Antidepressants: What the Numbers Are Telling Us
Antidepressant use has become a defining feature of modern mental healthcare. In the UK alone, an estimated 8.7 million people are currently taking antidepressant medications, reflecting both the scale of mental health need and the central role pharmacological...

Phacilitate Unites CGT Community for New Era Defined by Collaboration
Phacilitate announces the return of Advanced Therapies Week (ATW) for its 22nd year, reinforcing its status as one of the most established and influential cell and gene therapy (CGT) conferences in the United States and among the most recognized globally. In...

Natural Product Drug Discovery Returns With New Anti Cancer Insights
Scientists have uncovered how plants produce mitraphylline, a rare natural compound with demonstrated anti cancer properties, shedding new light on the biological machinery behind one of nature’s most complex chemical products. By identifying the key enzymes involved...

Biotech’s Funding Winter is Over, but M&A Will Remain Selective
Biotech is showing early signs of life after a prolonged funding winter, but for many early-stage companies, conditions remain unforgiving. Years of low valuations, high borrowing costs and regulatory uncertainty have reshaped investor behaviour, with capital scarce...

Thousands Benefit From New Treatment for Advanced Prostate Cancer
NICE recommends life-extending daily pill which offers hope for people who cannot take standard treatment. Thousands of people living with advanced prostate cancer will have access to a life-extending new treatment that can be taken at home from today (Friday, 23...
Featured Articles
Further News

A Career in Life Sciences: Pathways, Possibilities and Support
The life sciences sector sits at the intersection of discovery, technology and human impact. From developing new medicines and diagnostics to advancing environmental sustainability and digital health, careers in life sciences offer the chance to work on problems that...
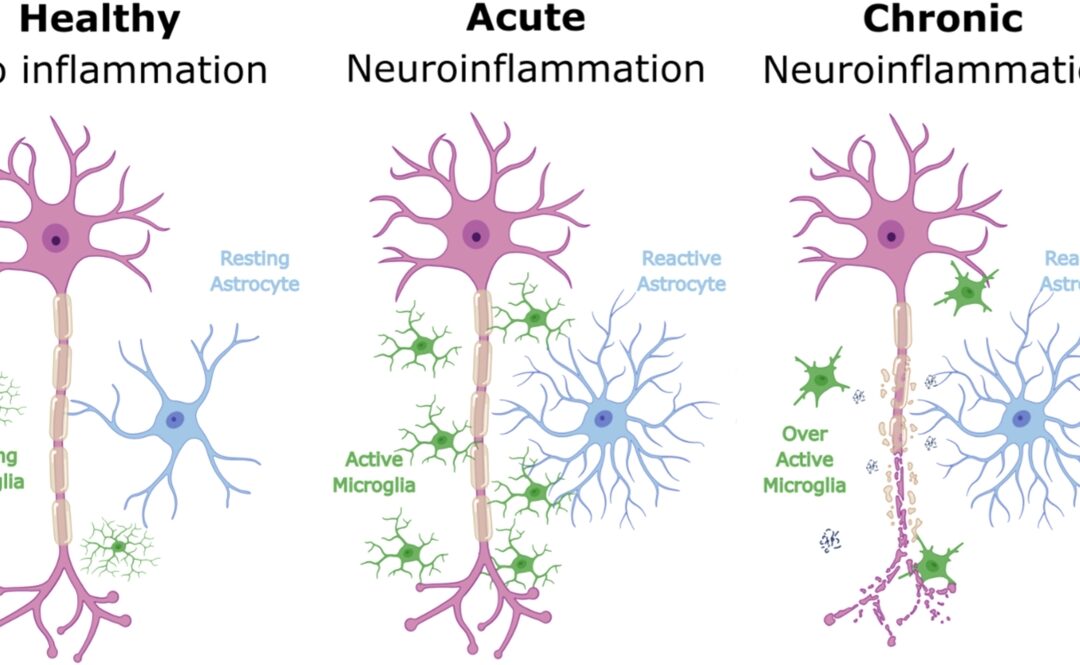
MRI Study Links Inflammation in Brain Reward Centre to Depression Severity
New research suggests that inflammatory and microstructural changes in a key brain reward region may differ between chronic depression and acute depressive symptoms, offering fresh insight into the biological mechanisms underlying the condition. In a large scale...
‘The World Has Not Yet Learned From the COVID Crisis’ Experts Warn
New research published (22 January 2026) in The Lancet Psychiatry warn that lessons have not been learned from the pandemic when it comes to mental health. The researchers recommend that mental health should be treated as a core consideration when it comes to pandemic...
Recovery as Signal Engineering: How Stress Upgrades Human Physiology
For decades, recovery has been framed as the absence of stress. Rest, relaxation, sleep, and passive modalities have been treated as the antidote to physiological load. While these elements matter, they represent only a fraction of how biological systems actually...

Novo Nordisk and Aspect Biosystems Expand Diabetes Collaboration
Novo Nordisk and Aspect Biosystems expand collaboration to accelerate curative diabetes therapies The landscape of regenerative medicine has shifted significantly following the announcement that Novo Nordisk and Aspect Biosystems are entering a comprehensive new phase...

Why Every Business Leader Should be Assessing Their Brain in 2026
As a business leader, we reflect on so much of our performance but not the thing that's driving it: our brains. Yet it's our brain anatomy which is truly individualised and unique to each business owner. Without it, our business cannot run. As a cognitive health...

GSK Makes $2.2 Billion Bid for RAPT Therapeutics
GSK has agreed to acquire US biotech company RAPT Therapeutics in a deal valued at approximately 2.2 billion US dollars, strengthening its position in immunology and signalling a major push into the emerging food allergy treatment market. The acquisition centres on...
What is the Metabolic Reset Diet – The Science
The science, the promise, and the pitfalls The idea of a “metabolic reset” has gained traction across wellness media, social platforms, and weight management programmes. The premise is appealing: that metabolism can be “retrained” or “rebooted” through short-term...

Obesity Redefinition Studies Challenge Longstanding Clinical Standards
For decades, obesity has been defined and diagnosed primarily using body mass index (BMI), a simple ratio of weight to height. While BMI has been widely adopted for its convenience and scalability, a growing body of research now suggests that it may be an incomplete...
Functional Gut Clinic Launches GI Clinical Trials Division
The Functional Gut Clinic (FGC), the UK’s leading provider of specialist gastrointestinal (GI) diagnostics, today announced the launch of its dedicated GI Clinical Trials establishing the organisation as the country’s first boutique, end‑to‑end clinical research...

Japan’s iPSC Breakthrough: A New Hope for Spinal Cord Injury Repair
Spinal cord injuries remain one of the most challenging conditions in modern medicine. Damage to the spinal cord often leads to permanent paralysis, loss of sensation, and a dramatic decline in quality of life. For decades, treatment options have been limited to...
Life Extending Prostate Cancer Drug to be Offered to Thousands in England
Thousands of men in England with advanced prostate cancer are set to gain access to a new life extending treatment following a decision by the National Institute for Health and Care Excellence. The move marks a significant development in the management of one of the...
Further News
Want to Be Part of the Next Chapter in Life Sciences Media?
LifeScienceDaily.news is building a new kind of platform where innovators, researchers, and industry leaders tell their own stories and shape the narrative of what’s next in life sciences. Share your story and lead the conversation.
Don’t wait for the media to pick up your story. Be the media.
See More Articles
Want to Be Part of the Next Chapter in Life Sciences Media?
LifeScienceDaily.news is building a new kind of platform where innovators, researchers, and industry leaders tell their own stories and shape the narrative of what’s next in life sciences. Share your story and lead the conversation.