Pharmaceutical approaches to ALS remain sparse and “hard-to-develop”. Riluzole (approved 1995) and edaravone (IV in 2017, oral in 2022) only offer modest benefits.
More recently, Biogen‘s Qalsody (tofersen) was approved for SOD1-mutant ALS.
But a new wave of new therapeutics is under development, including Spinogenix, Inc.’s Exciting Small Molecule, SPG302.
What is SPG302?
- A first-in-class “synaptic regenerative” small molecule; as a once-daily oral pill. Designed to restore and protect synapses
- It was granted Orphan Drug Designation by both the FDA and European Medicines Agency, with preclinical support from The National Institutes of Health/ Department of Defense (DoD) CAP
Phase 2a Results (6-month study)
- SPG302 was well tolerated with no treatment-related serious adverse events over six months.
- 82% of patients on SPG302 had stable or improved ALSFRS-R trajectory over 6 months.
- SPG302-treated patients showed about a 76% slower rate of functional decline over 6 months (vs historical datasets)
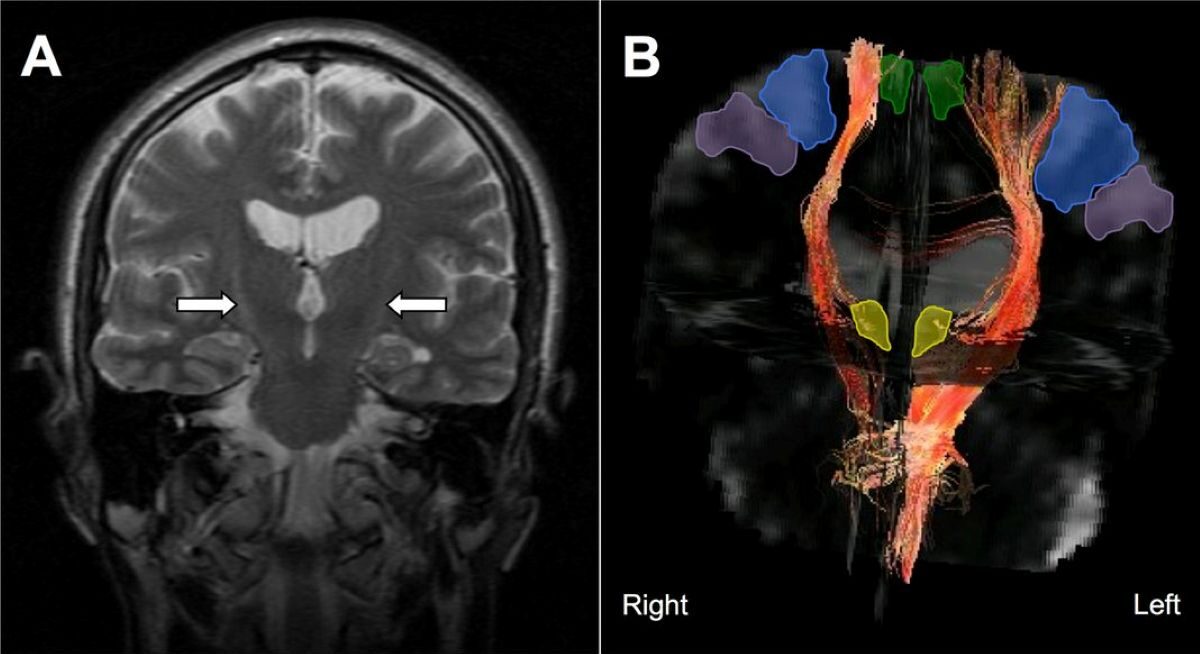
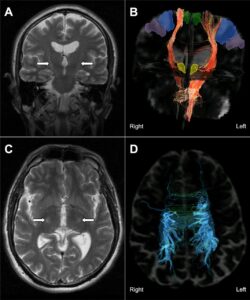
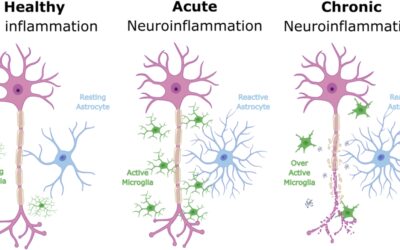
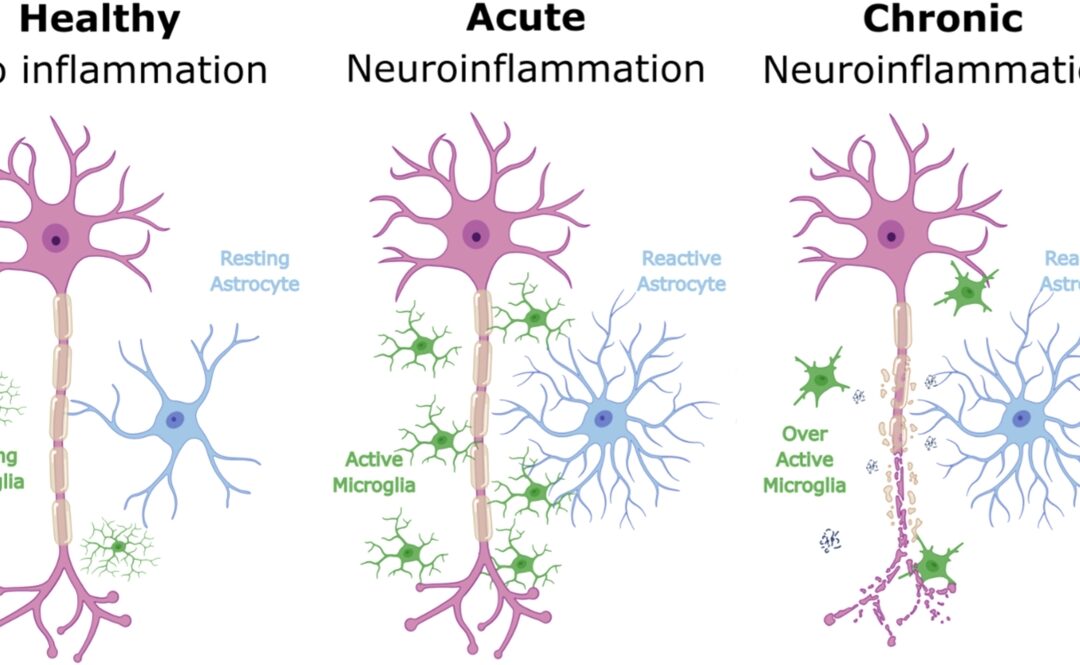
- EEG signals suggested improved brain activity patterns associated with ALS.
Congratulations to the amazing team involved, and it was great to hear their CEO (Stella Sarraf, PhD), and merit cudkowicz confirm the commitment to an expanded access program for patients ineligible for their current studies!
ALSResearch Biotech SynapticBiology ClinicalTrials Expandedaccess Neurology