Miraculous Progress for 3-Year-Old After Ground-Breaking Gene Therapy for Hunter Syndrome
A three-year-old boy, Oliver Chu, has astounded doctors after becoming the first person in the world with Hunter syndrome also called MPS II to receive a pioneering gene therapy. His remarkable improvement is offering fresh hope for a disease long considered devastating and untreatable.
A Family’s Fight Against a Fatal Inherited Disorder
Hunter syndrome is a rare, inherited condition caused by a deficiency of the enzyme iduronate-2-sulfatase IDS, leading to the accumulation of complex sugar molecules in the body. Over time, this build-up causes progressive damage to organs and, in severe cases, to the brain. Without treatment, many affected children face shortened lifespans, often succumbing before their twenties.
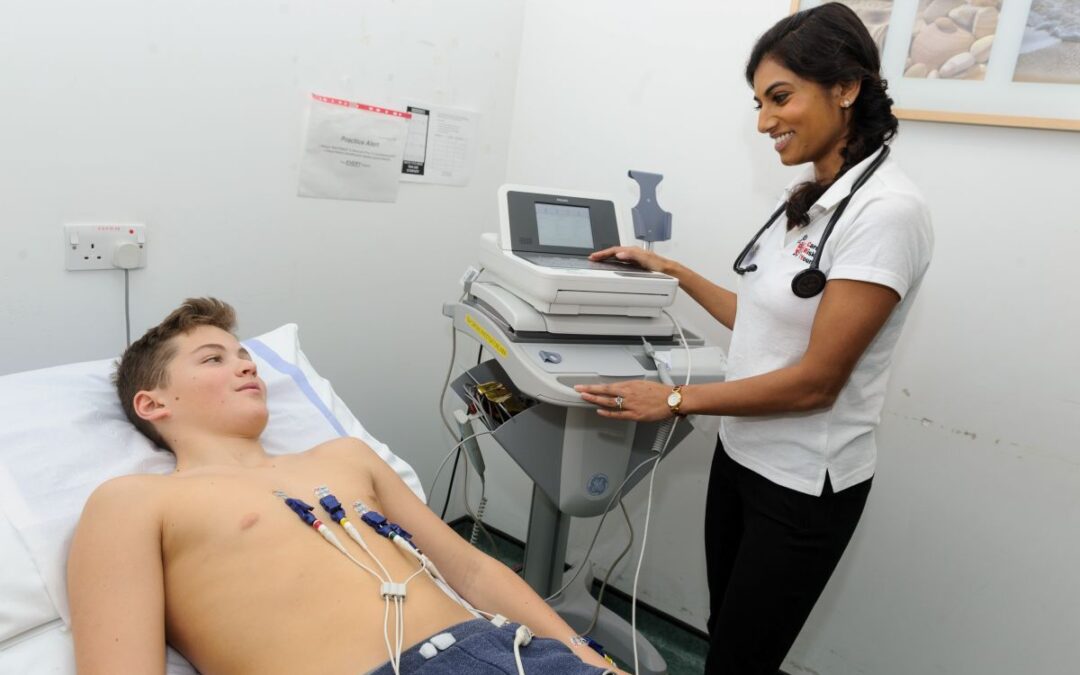
Oliver’s parents, Ricky and Jingru Chu, first noticed worrying signs when he was around two years old. Diagnosed in April, Oliver was one of only a handful of boys in the world selected for a first-of-its-kind cell-based gene therapy trial led in Manchester. To qualify, his stem cells were harvested, modified in the laboratory, and re-infused in a delicate procedure earlier in 2025.
How the Therapy Works
At the heart of this experimental treatment is gene therapy: Oliver’s stem cells were removed and sent to a specialised lab, where scientists inserted a functioning copy of the IDS gene using a modified virus. These genetically corrected stem cells were then infused back into his body, with the aim of producing the missing enzyme.
Crucially, the therapy was designed to address one of the biggest challenges in MPS II: how to restore enzyme production in the brain. Researchers engineered the gene to direct production not just in the body, but also in the brain, effectively enabling enzyme delivery across the blood-brain barrier.
Early Evidence of Success
Just a few months after the treatment, Oliver’s parents began to notice changes. By May 2025, his mobility, speech, and engagement had noticeably improved. His mother, Jingru, shared that Oliver seemed “brighter and healthier,” and that his weekly infusions of the missing enzyme previously a lifeline had been stopped. He was reportedly making the enzyme on his own. His father, Ricky, added:
“He’s like a completely different child … he’s running around everywhere, he won’t stop talking.”
Prof Simon Jones, one of the clinicians leading the trial, expressed cautious optimism:
“Before the transplant, Ollie didn’t make any enzyme at all and now he’s making hundreds of times the normal amount. More importantly, we can see he’s improving — he’s learning, he’s got new words, and he’s moving far more easily.”
Despite the early excitement, Prof Jones emphasised the need for long-term follow-up:
“We need to be careful and not get carried away … but things are as good as they could be at this point in time.”
A Global First, and Just the Beginning
Oliver is the first of five boys worldwide enrolled in this trial, which spans several countries. The remaining participants come from the United States, Europe, and Australia. All will be followed closely for at least two years to assess safety, enzyme production, cognitive development, and long-term outcomes.
If results remain positive, the hope is to licence the therapy for wider use potentially transforming the outlook for boys with MPS II globally. Indeed, the same approach may someday be applied to other related lysosomal disorders, such as MPS I Hurler syndrome or MPS III Sanfilippo syndrome.
Where This Fits in the Larger Gene Therapy Landscape
Oliver’s treatment exemplifies the promise of gene therapy for MPS II a field that is also being advanced by companies developing alternative approaches. For example, REGENXBIO is developing RGX-121, an adeno-associated virus-based gene therapy that recently entered priority review by the FDA. That approach aims to deliver the IDS gene directly to patients’ cells in a single treatment.
Meanwhile, other companies are pushing forward with enzyme therapies, small molecules, and future cell-and-gene options. This is part of a broader surge of innovation in MPS II treatment, driven by advances in both science and regulatory frameworks.
Unanswered Questions and Risks
While Oliver’s progress is remarkable, challenges remain. The long-term durability of his enzyme production is unknown, as is the risk of immune reactions or other complications from the modified stem cells. Scientists will be watching carefully for how well these gene-corrected cells engraft, proliferate, and continue to secrete the IDS enzyme over years.
Regulatory approval will also be complex. As with other one-time gene therapies, authorities will need to weigh not just early efficacy but also long-term safety, manufacturing consistency, and cost. Real-world rollout would raise questions about access and affordability, given the enormous expense of developing and delivering such advanced therapies.
Why It Matters
For Oliver and his family, the therapy has already changed his life: what was once a relentless schedule of hospital visits and infusions may be replaced by childhood milestones, play, and growth. For the broader MPS II community, his case represents a powerful proof of concept that gene therapy could offer more than just symptom management. It could offer a real shot at long-term stability, or even a cure.
If successful, this therapy would join a growing number of gene- and cell-based treatments reshaping the landscape for rare genetic diseases. It underscores how scientific ambition, clinical innovation, and patient courage can align to reshape futures.