Engasertib Trial Offers New Hope for Patients with Hereditary Hemorrhagic Telangiectasia
A recent clinical study has delivered encouraging results for people living with hereditary hemorrhagic telangiectasia, a rare genetic vascular disorder that causes chronic and often severe bleeding. The investigational drug engasertib has shown meaningful reductions in bleeding symptoms, offering renewed hope for a patient community long without any approved disease modifying treatments.
Understanding HHT and Its Impact
Hereditary hemorrhagic telangiectasia, also known as Osler Weber Rendu syndrome, is an inherited condition that leads to the development of fragile blood vessels prone to rupture. These include telangiectases and arteriovenous malformations that may form throughout the skin, mucous membranes and internal organs. The disease affects multiple systems and can cause recurrent nosebleeds, gastrointestinal bleeding, chronic anemia and in some cases serious complications involving the liver, lungs or brain.
Because the condition varies widely in severity, some individuals experience mild symptoms while others require frequent medical interventions such as cauterisation procedures, iron infusions or blood transfusions. Historically, most treatment approaches have focused on managing symptoms rather than addressing the root mechanisms that drive the vascular abnormalities.
Engasertib Achieves Promising Results in Clinical Evaluation
The investigational drug engasertib, developed by Vaderis Therapeutics, recently completed a controlled study involving 75 participants. The trial was designed to assess whether the oral therapy could reduce bleeding severity and improve quality of life for individuals with hereditary hemorrhagic telangiectasia.
Over a 12 week treatment period, participants receiving engasertib experienced meaningful improvements compared with those on placebo. The frequency of nosebleeds declined substantially in the highest dose group and the duration of individual bleeding episodes also decreased. Patients reported feeling better overall, with more than half of those receiving the top dose describing significant improvement.
The drug was generally well tolerated. The most common side effects included mild rash and temporary increases in blood sugar levels, while serious adverse events occurred at rates similar to the placebo group.
Dr Hanny Al Samkari, a leading investigator on the study, noted that the findings represent a major step forward for patients who currently have limited treatment choices. He stated that the results indicate engasertib could offer a meaningful new option for those affected by this lifelong condition.
A New Therapeutic Approach for HHT
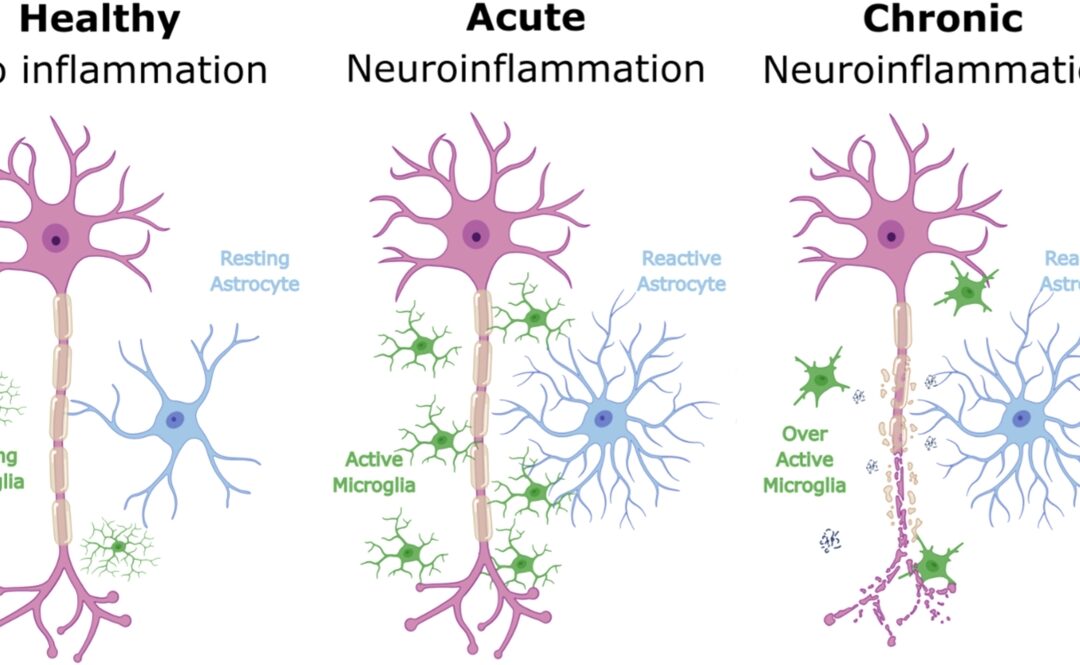
Engasertib works by modulating the AKT signalling pathway within cells. This pathway is known to be overactive in hereditary hemorrhagic telangiectasia as a result of gene mutations involved in vascular development. By adjusting this pathway, the therapy aims to stabilise malformed blood vessels and reduce their tendency to bleed.
This mechanism differs from previous systemic therapies that attempted to suppress the formation of new blood vessels. While some of these off label treatments have shown modest benefit in individual cases, they have not consistently produced durable results and may cause significant long term side effects.
If further research confirms the benefits of engasertib, it may represent a true disease modifying therapy rather than a purely symptomatic one. This would mark a substantial shift in the management of hereditary hemorrhagic telangiectasia.
Emerging Interest in Targeted HHT Therapies
The progress of engasertib comes at a time of growing interest in developing targeted treatments for the disease. Other investigational programmes are also underway, including therapies designed to correct errors in the signalling pathways that influence the formation and stability of blood vessels. Patient advocacy groups and research networks have played a significant role in advancing these efforts by establishing standardised clinical outcome measurements and supporting natural history studies to better understand disease progression.
These initiatives aim to build a stronger evidence base that will help accelerate the development and approval of new therapies.
Future Considerations
Although the recent trial results mark an important milestone, researchers emphasise that larger and longer studies will be required to fully understand the long term safety and effectiveness of engasertib. Questions remain about how well the therapy will work across the diverse patient population and whether it can reduce the risk of severe internal complications.
The path toward approval will require continued collaboration among clinicians, researchers, patient organisations and regulatory agencies. Nevertheless, the initial findings have generated cautious optimism within the hereditary hemorrhagic telangiectasia community.
For decades, individuals with hereditary hemorrhagic telangiectasia have faced a limited set of treatment options centred primarily on managing symptoms. The encouraging results from the engasertib trial represent a potential turning point. If future studies confirm its benefit, engasertib may become the first therapy specifically designed to modify the underlying disease process, signalling a new era in the care of this rare and challenging condition.