Pharvaris Announces Positive Phase 3 Results for Deucrictibant as On Demand HAE Treatment
Pharvaris has announced positive topline data from its global Phase 3 RAPIDe 3 study, confirming that the oral bradykinin B2 receptor antagonist deucrictibant shows strong potential as an on demand therapy for acute hereditary angioedema (HAE) attacks. The results have generated significant optimism within the rare disease community and represent an important step toward a more convenient and effective option for treating HAE.
Key Results: Rapid Relief and High Efficacy
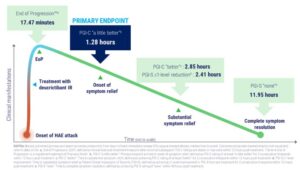
In the RAPIDe 3 study participants treated with deucrictibant experienced substantially faster symptom improvement compared with placebo. The median time to onset of symptom relief was 1.28 hours, in contrast to more than 12 hours for those receiving placebo. All 11 secondary efficacy endpoints reached statistical significance.
Other notable findings included a median time to end of progression of 17.47 minutes compared with 228.67 minutes for placebo, a significant reduction in time to symptom relief based on patient reported measures, and a median of 11.95 hours to complete symptom resolution compared with more than 24 hours for placebo. Additionally, 83 percent of attacks required only a single dose and more than 93 percent required no rescue medication.
The safety profile was favourable with no treatment related serious adverse events and no discontinuations. Efficacy and safety outcomes were consistent across HAE subtypes, severities, anatomical locations of attacks, and among both adolescents and adults.
Significance: Convenience Meets Efficacy
For people with HAE attacks can be unpredictable and potentially life threatening, particularly when affecting the airways. Many current on demand therapies require injections and can be challenging for some patients to administer during emergencies. An oral capsule that acts rapidly and reliably may significantly improve treatment accessibility and adherence.
Clinical experts involved in the study emphasised the importance of having an effective and well tolerated acute therapy that patients can use quickly and confidently when symptoms begin. Pharvaris leaders noted that the comprehensive results from RAPIDe 3 underscore deucrictibant’s potential to meet an important unmet need for the HAE community.
What Comes Next: Regulatory Filings and Future Development
Following these positive results, Pharvaris plans to submit a New Drug Application to the United States Food and Drug Administration in the first half of 2026 for the immediate release oral capsule. Meanwhile the company continues its CHAPTER 3 Phase 3 study evaluating a once daily extended release formulation for preventive treatment. Topline results from CHAPTER 3 are expected in the second half of 2026.
Pharvaris is also preparing a global pivotal Phase 3 study investigating deucrictibant in acquired angioedema due to C1 inhibitor deficiency which could broaden its therapeutic reach beyond hereditary angioedema.
Implications for the HAE Community
If approved deucrictibant could become a major advancement in on demand care for hereditary angioedema. The ability to offer an oral therapy with rapid onset single dose effectiveness and strong tolerability would represent a meaningful improvement in quality of life for many patients. It may also simplify clinical management and further validate the role of bradykinin B2 receptor antagonism as a key mechanism in HAE treatment.