In a ground breaking advance in reproductive biology, researchers in the United States have for the first time created early-stage human embryos by converting DNA from skin cells into functional eggs and then fertilising them with sperm. Though still at an experimental stage, the work marks a striking step toward new infertility treatments, while raising profound ethical and safety questions.
From Skin to Egg to Embryo: The Experimental Journey
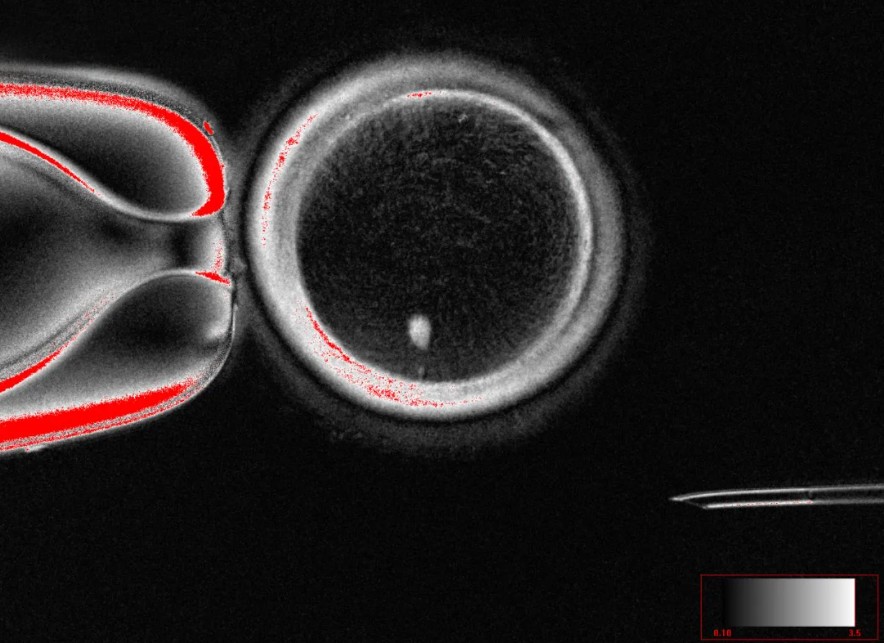
The research, led by scientists at Oregon Health & Science University (OHSU), makes use of a technique in which the nucleus of an adult skin cell is transplanted into a donor egg cell stripped of its own nucleus. This process effectively transfers the donor’s genetic material into the egg environment, initiating a series of events akin to natural egg maturation. After fertilisation with sperm, some of these engineered eggs developed into early-stage embryos.
A key technical hurdle is ensuring that the egg ends up with a single set of chromosomes, as is standard in natural gametes, rather than the normal diploid complement of the skin cell. To manage this, the researchers employed a procedure—recently termed “mitomeiosis”—which induces the cell to discard half of its chromosomes, mimicking the reductional division that normally occurs in meiosis.
In their experiments, the team produced 82 engineered eggs and fertilised them with sperm. Of those, about 9 per cent proceeded to the blastocyst stage (around six days post-fertilisation), a common benchmark in fertility treatments. Unfortunately, all of the resulting embryos showed substantial chromosomal abnormalities, making them unsuitable for further development or implantation.
OHSU’s Shoukhrat Mitalipov, a leading figure in nuclear transfer and stem cell research, has expressed that while the work is not yet safe or efficient for clinical application, it serves as an important proof of concept. Paula Amato, also a co-author, emphasised that chromosome pairing in these embryos is currently random and often incorrect—one major barrier to creating healthy embryos.
Implications for Infertility and Beyond
One of the most tantalising potential applications of this technology lies in treating infertility, especially in cases where individuals have no viable eggs, for example older women or those whose eggs have been damaged by chemotherapy. The technique might eventually allow such individuals to have genetically related offspring using their own somatic cells. It could also open pathways for same-sex couples to have children genetically related to both partners, by converting a skin cell from one partner into an egg and fertilising it with the other partner’s sperm.
Beyond reproduction, the approach could provide researchers with new tools to probe human embryo development, genetic disease formation, or developmental defects—areas that are otherwise difficult to study because of the scarcity of donated human embryos.
Still, experts caution that the road from scientific demonstration to safe clinical use is long and complex. Efficiency must improve dramatically, chromosomal integrity must be assured, and long-term developmental viability remains untested. Moreover, U.S. regulations currently bar the use of genetically modified embryos for implantation in patients, confining such research to laboratory settings.
Ethical, Regulatory and Safety Considerations
As with any venture into engineered human embryos, this development renews longstanding ethical dilemmas around human germline manipulation, embryo status, and possible so-called designer applications. Some ethicists warn of slippery slopes toward embryo farming or selection beyond medical necessity. Others urge robust public dialogue, bioethics in reproduction, regulatory oversight, and clear boundary setting before any clinical translation.
Concerns also extend to safety: the abnormal chromosome complements in all tested embryos underscore that current versions are profoundly imperfect. Introducing embryos with undetected chromosomal defects into a uterus would carry serious risks. Because of these risks, the authors and commentators uniformly agree that it will likely take a decade or more of further research to approach a point where clinical trials might be contemplated.
The advancement also raises questions about oversight: how to regulate engineered embryos, whether to permit commercial use, how to ensure equity of access, and how to guard against misuse. Some jurisdictions may prohibit such interventions entirely, while others may adopt cautious pathways.
Relationship to Prior Embryo-Model Systems
This accomplishment differs from earlier efforts to create embryo-like structures, known as blastoids, derived from stem cells or reprogrammed skin cells. Those models resemble early embryos but are non-viable and lack full developmental potential. In contrast, the OHSU method actually produces fertilised embryos (albeit highly abnormal) by combining engineered eggs and sperm. However, it remains a leap from these proof-of-concept embryos to any implantation or further development.
Outlook and Next Steps
While this advance is a major scientific milestone, its promise is tempered by the distance still to travel. The key technical challenges include dramatically improving the proportion of healthy embryos, ensuring correct chromosomal segregation and pairing, and verifying long-term developmental viability and safety in animal or model systems.
Parallel to scientific development, ethical and regulatory frameworks will need to evolve. Public engagement and oversight will be essential to ensure responsible use, transparency and prevent misuse.
If the challenges can be overcome over the coming years, this technology could transform reproductive medicine and reshape the possibilities for treating infertility, studying human development, and understanding genetic disorders. For now, it stands as a powerful proof of principle—and a reminder of both the promise and perils of human embryo engineering.