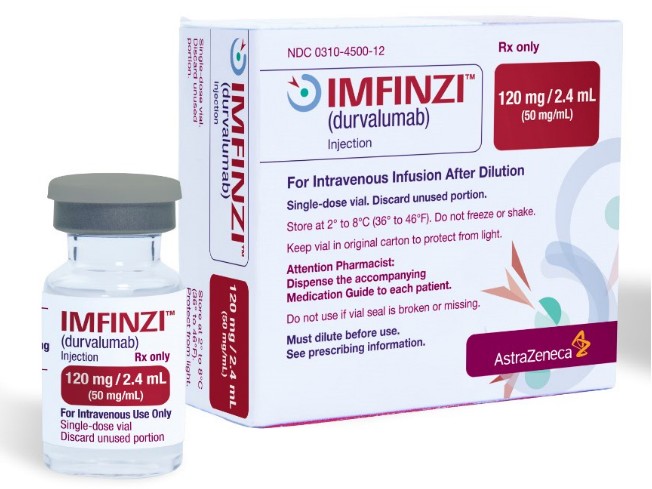
Durvalumab approved in the US as first & only perioperative immunotherapy for patients with early gastric & gastroesophageal cancers!
The FDA approved Durvalumab (Imfinzi) in combination with Fluorouracil, Leucovorin, Oxaliplatin, and Docetaxel (FLOT) chemotherapy as neoadjuvant & adjuvant treatment, followed by a single agent (Durvalumab) to treat adults with respectable, early-stage, & locally advanced gastric or gastroesophageal junction adenocarcinoma (GC/GEJC).
MATTERHORN Phase III trial evaluated the safety & efficacy of the treatment in a total of 948 patients with previously untreated & resectable GC/GEJC that were randomly assigned 1:1 to receive Durvalumab and FLOT or placebo and FLOT.
MATTERHORN trial showed a 29% reduction in the risk of progression, recurrence or death and a 22% reduction in the risk of death for the IMFINZI regimen vs. chemotherapy alone.
An estimated 69% of patients treated with the IMFINZI-based regimen were alive at three years compared with 62% in the FLOT-only arm, & with longer follow-up, the OS curves showed continued separation, signaling a greater magnitude of benefit over time for the IMFINZI-based regimen.
The approval follows Priority Review by the Food and Drug Administration (FDA) and is based on event-free survival (EFS) and overall survival (OS) data from the MATTERHORN Phase III trial.
“This approval ushers in a new clinical paradigm for patients with early GC/GEJ cancers, with [Durvalumab] plus FLOT delivering a durable survival benefit that increases over time. As the third US approval for a perioperative [Durvalumab]-based regimen, this milestone further validates the perioperative approach and underscores our focus on bringing novel treatments to early-stage cancers where cure is the goal,” Dave Fredrickson, executive vice president, oncology hematology business unit, AstraZeneca, said in a news release.